Characteristics of Organic Compounds Which Give Their Uses
Most organic compounds are nonpolar and thus do not mix with polar molecules like water. Esters are compounds that can give flavor taste and smell to some foods and perfumes.
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry
Used for curing fungal infections.

. Alcohols such as ethanol and propanol come under the category of organic compounds. And used as main ingredient in liquors Kerosene fuel for lamps and portable cooking stove acetone used to remove nail polish LPG fuel for gas stove and cars acetic acid. Acetone is used to synthesize methyl methacrylate.
These compounds are combustible in nature. Sulphur iodine and many other organic compounds can be dissolve in it. Uses of Organic Compounds.
1preparation of wines and alcohols using fermentation sugar ethanol 2. It is also used as an antiseptic in medical wipes and hand sanitizers. Most properties of the compounds are decided.
Observe the materials and write the phase and odor of the materials on the. Aspirin a widely used medicine also contains carboxylic acid. Used for gas stoves.
Used for making soaps using hydrolysis of esters using NaOH. Ethanol is the clear colorless liquid which is widely used in the manufacturing of beer whiskey wines and so on. Though they are present almost everywhere the most common uses are.
The bonds formed between the carbon and hydrogen atoms to form a hydrocarbon are very strong and the resulting compound is often essential to living things. Organic compounds are those who chemical formula contains at least one carbon atom and often contain a hydrogen atom as well. Activities questions directions exercises and discussions are carefully stated for you to understand each lesson.
Used for removing nail Polish. Organic compounds have the following general characteristics. Organic compounds have various uses in everyday lives.
Some alkanes or mixtures of alkanes are generally used as fuels. Properties of Common Organic Compounds. Proteins- silk wool casein in different food etc.
The general characteristics of Organic Compounds are. Carbon Hydrogen and nitrogen. Test tube according to the liquid it contains.
I Organic compounds are generally covalent and hence they do not dissolve in water polar solvents but dissolve in organic solvents non-polar solvents such as ether alcohol benzene etc. Which of the following are correct examples of the properties or uses of organic compounds. The chemical property tells us how it reacts to the development of new usable materials with intense properties such as the ductility to be drawn in a wire to be used for.
What do you think are the characteristics of the materials which give their uses. Making perfumes and essence for soaps. Carboxylic acid which is also an organic compound comes into use in the pharmaceutical industry.
Alcohols are all toxic to humans. Used in alcohol and liquors. In the laboratory acetone is used as a polar aprotic solvent in a variety of organic reactions.
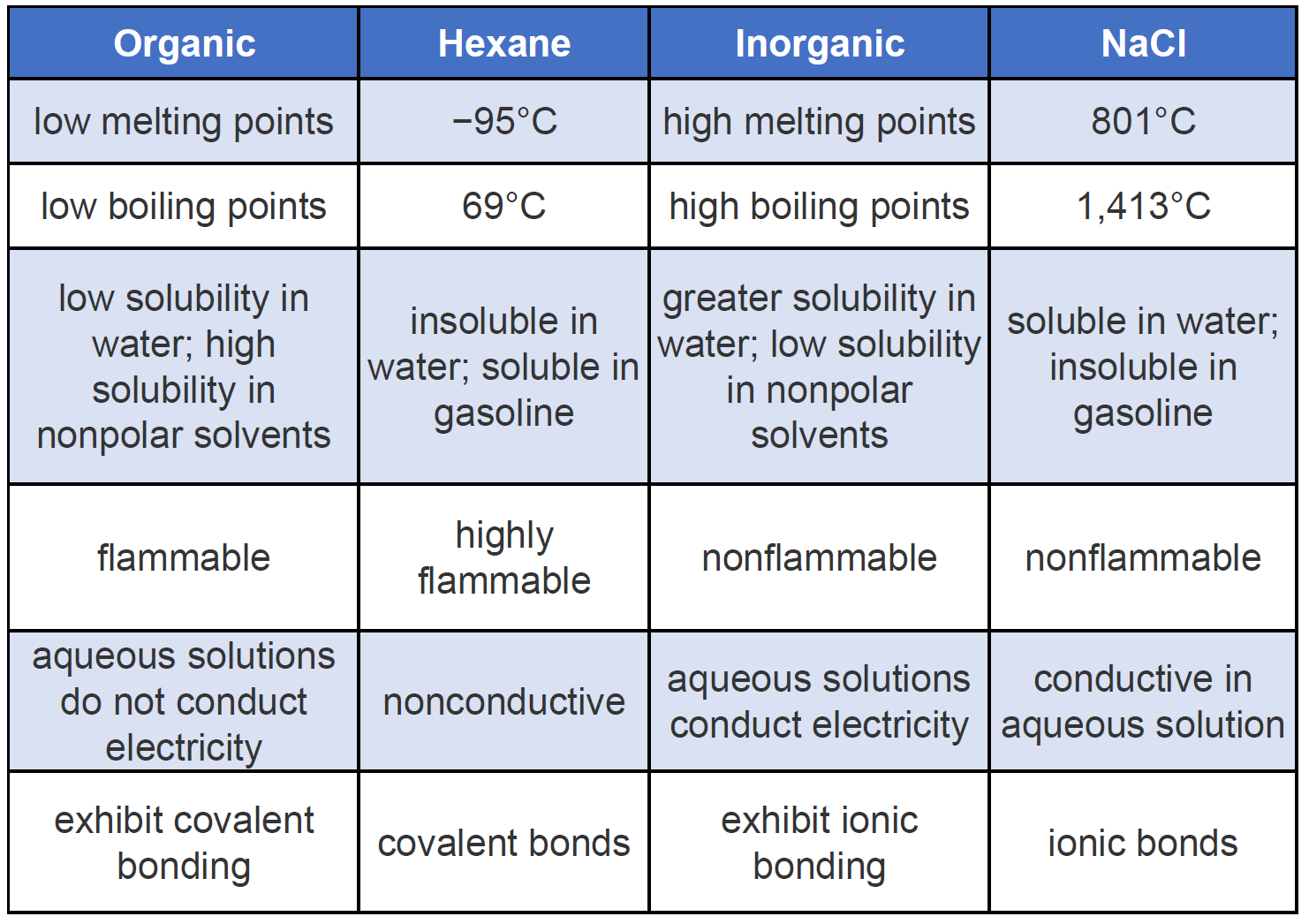
The properties of organic compounds are very different from the properties of inorganic compounds that you have been using up to this point. Alcohol has many uses including ethanol consumption or use as an antiseptic. Each SLM is composed of different parts.
Ii Due to maximum catenation and tetravalency of carbon they have tendency to form long open and. Making perfumes and essence for soaps. Organic compounds include complex structures and high molecular weights.
Hydrocarbons a category that includes groups of molecules called alkanes alkenes and arenes according to their structure are made up entirely of hydrogen and carbon and release a great deal of energy when burnt making hydrocarbons such as propane butane and. Such as Carbohydrates- cellulose sugar starches etc. Choose all that apply.
In this lab you will explore some of the differences in the properties of organic vs. The following are the uses of the organic compounds listed above. 2 Testing the viscosityof the materials.
It is also useful in the manufacturing of acetate acetone and esters used in various industries. Low melting points and boiling points in comparison to the inorganic compounds. Used for making soaps using hydrolysis of esters using NaOH.
Properties of Organic Compounds. These common organic compounds are very important because they have many uses at home and in the industry. Organic acids and bases are less stronger and thus they.
A carbon-carbon single covalent bond has energy of 346 kJ mol-1. Most organic compounds are stable because of the strong carbon-carbon bonds. Used for fuel in gas stove.
Some organic categories have interesting properties that people often encounter in their daily lives. Alcohol is an addictive psychoactive drug widely used amongst adults and teenagers to get high. The chemical properties and the intensive properties of the material will be the determining factor for their use.
Compounds Uses Compounds Uses Gasoline fuel for vehicles ethanol disinfectant. Acetone is a good solvent for many plastics and some synthetic fibers. These are soluble in organic solvents and mostly insoluble in water.
Gasoline Used as fuel for vehicles. Place 15 ml of each liquid in the four 4 identical test tubes and label each. 1preparation of wines and alcohols using fermentation sugar ethanol 2.
Compounds used as medicinal products are most usually organic compounds sometimes divided into large groups of small organic molecules eg atorvastatin fluticasone clopidogrel and biologics infliximab erythropoietin insulin glargine the latter more widely used as protein medicinal products. Fats and oils- cottonseed soyabean oil butter etc. Alkaloid- quinine morphine etc.
Used as fuel for Lamps. Alcohols are all toxic to humans. Used to remove paint.
Organic compounds contain carbon and hydrogen combined with other elements namely oxygen nitrogen phosphorous sulfur and halogens fluorine chlorine bromine and iodine Ethyl alcohol acetone gasoline napthalene acetic acid vanillin acetylene and esters are just a few examples of many useful organic compounds. Since they have a covalent nature they do not ionize in solution and are non-conductors of electricity. Though they are present almost everywhere the most common uses are.
Organic compounds have various uses in everyday lives. This high energy indicates a strong bond. Grade 9 Science Module.
In the rubber industry it serves as a coagulating agent. About a third of the worlds acetone is used as a solvent and a quarter is consumed as acetone cyanohydrin a precursor to methyl methacrylate. The presence of a covalent bond renders certain characteristics to the organic compounds.
Organic compounds are found in large number in nature and they are well known for their uses. Mostly depend on only three elements. This Self-Learning Module SLM is prepared so that you our dear learners can continue your studies and learn while at home.

Difference Between Organic And Inorganic Compounds Definition Structure Properties Chemistry Lessons Chemistry Education Teaching Chemistry

General Introduction To Organic Compounds Properties Uses With Videos
No comments for "Characteristics of Organic Compounds Which Give Their Uses"
Post a Comment